Understanding The Increasing Importance of Non-invasive Biosensors
 |
| Non-invasive Biosensors |
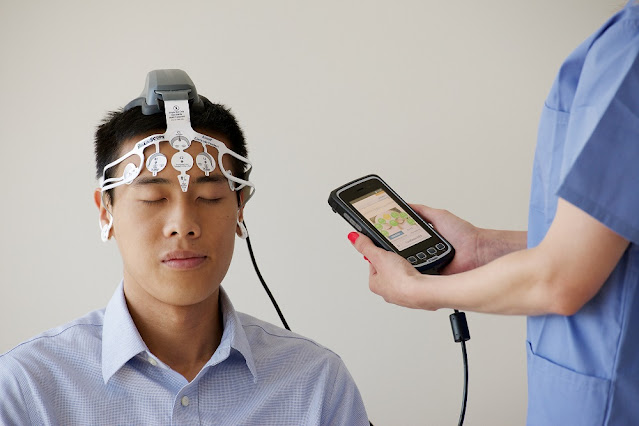
The evolution of non-invasive biosensors
Non-invasive biosensors have come a long way since their conception in the
1960s. Originally proposed as a diagnostic tool to monitor vital signs and
detect biomarkers from a distance without drawing blood or breaching the skin,
early prototypes were primitive and lacked accuracy. Over the past few decades,
advances in materials science, microfabrication, signal processing, and
miniaturization have enabled the development of highly sensitive and selective
biosensors that can reliably perform in vivo, continuous monitoring.
Major advancements in sensor technologies in the 2000s built upon knowledge
gained from genomics and proteomics research. Novel nanomaterials like
graphene, carbon nanotubes, nanoparticles strengthened biosensor performance.
Microfluidic platforms combined with precision manufacturing allowed for
multi-analyte detection on a single wearable strip or patch. Optical,
electrochemical and acoustic sensing mechanisms became more refined for
deriving clinically relevant data from sweat, saliva, breath or other
biofluids.
The wireless communication capabilities of biosensors also expanded
tremendously. Ultra-low power Bluetooth and cloud connectivity permitted
real-time transmission of vital signs to physicians and predictive algorithms.
This remote monitoring facilitated proactive healthcare interventions instead
of traditional reactions to symptoms. Concurrently, miniaturization efforts
through system-on-chip designs and flexible electronics enabled discrete,
inconspicuous form factors suitable for long term implantation or integrated
with wearable garments.
Current impact and future outlook
Today, Non-Invasive
Biosensors have started
disrupting several areas of healthcare. Continuous glucose monitors help
diabetics better manage blood sugar levels without daily finger pricks.
Wearable heart and activity trackers notify users of potential health issues
detected through electrocardiograms and pulse oximetry. Sweat analysis tools
provide insight into drug adherence, hydration levels and physical exertion.
Breathalyzers allow for screening of biomarkers of disease and intoxication
with high accuracy. Such new form factors have improved both patient outcomes
and healthcare delivery models.
Going forward, the next generation of biosensors aims for even more intimacy
with the human body. Temporary tattoos, contact lenses, and
ingestible/implantable formats will enable seamless monitoring from within with
little disruption to daily lives. Multi-analyte capabilities will expand to
simultaneously track over a dozen biomarkers associated with multiple health
conditions. Continuous digital biomarkers derived from these data streams will
power more sophisticated artificial intelligence and machine learning
algorithms for predictive, preemptive and personalized care. Non-invasive
biosensing tools integrated into smart cities infrastructure may potentially
enable large-scale epidemiological modeling and public health surveillance as
well.
Regulatory and ethical considerations
While non-invasive biosensors promise revolutionary benefits, several
regulatory and ethical challenges will need addressing with their wider
adoption. Data privacy and cybersecurity are significant concerns due to the
sensitive physiological and health information continuously collected.
Standards must be established to ensure diagnostic-grade accuracy and prevent
misdiagnosis stemming from faulty sensors or algorithms. Affordability remains
a barrier, though mass production techniques may eventually reducing costs to
suitable levels.
Physician credentialing procedures may require adjusting as remote monitoring
shifts responsibility and liability. Informed consent processes must clearly
explain data usages and limitations upfront to build trust. Direct-to-consumer
platforms should involve medical oversight to avoid inappropriate
self-diagnosis or treatment. The potential for covert or mandated mass
surveillance will need safeguarding through legislation. Overall, a balanced
regulatory framework can help maximize the societal good from non-invasive
biosensors while mitigating privacy, security and clinical risks.
non-invasive biosensors are revolutionizing healthcare by
enabling continuous, objective physiological monitoring without drawing blood
or compromising comfort. Major technological advances now allow accurate,
wireless derivation of multiple clinical biomarkers from sweat, breath, tears
or other biofluids. Widespread adoption promises more proactive, predictive and
personalized care models through remote digital biomarkers and big data
analytics.
Going forward, even greater intimacy with the human body through novel form
factors will further minimize invasiveness. Addressing regulatory
considerations around data privacy, informed consent, accuracy and ethics will
be imperative to build public trust. When developed responsibly, non-invasive
biosensing tools have tremendous potential to transform global health outcomes
through low-cost, ubiquitous diagnostics and remote patient monitoring on an
unprecedented scale.
Get
more insights on – Non-Invasive
Biosensors
About Author:
Vaagisha
brings over three years of expertise as a content editor in the market research
domain. Originally a creative writer, she discovered her passion for editing,
combining her flair for writing with a meticulous eye for detail. Her ability
to craft and refine compelling content makes her an invaluable asset in
delivering polished and engaging write-ups.
(LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)

Comments
Post a Comment