The Rise Of Virtual Clinical Trials Have Historically Involved Bringing Patients
 |
| Virtual Clinical Trials |
Clinical trials have historically involved bringing
patients into physical locations like hospitals or clinics to participate. However,
with advancements in digital health technologies, a new model known as virtual
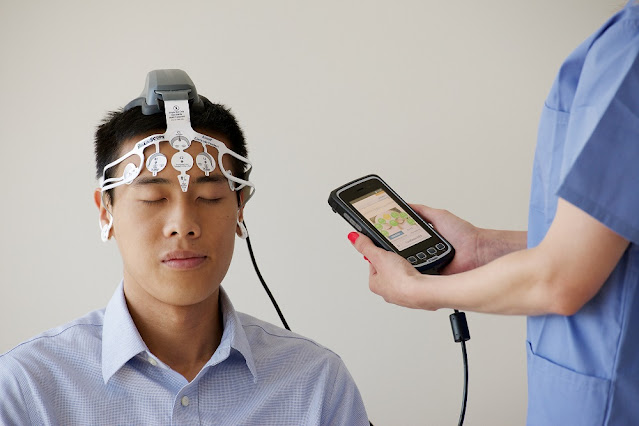
clinical trials is gaining traction. By utilizing remote patient monitoring
devices, mobile apps, and telehealth platforms, clinical trials can now recruit
and interact with participants entirely online without the need for in-person
visits.
This shift to virtual trials has accelerated significantly during the COVID-19
pandemic as travel restrictions and social distancing necessitated moving
trials online. faced major disruptions and delays at the start of the pandemic
when traditional in-person studies became impossible to conduct safely. Virtual
models enabled many trials to continue operating remotely. As a result,
regulators and pharmaceutical companies rapidly adopted new guidance supporting
virtual trials out of necessity.
Improving Access and Inclusion
A key benefit of Virtual
Clinical Trial is improved access and diversity in participant
recruitment. Geographic barriers are removed, allowing researchers to cast a
wider net and reach more potential candidates regardless of location. This can
help enroll adequate numbers of participants more quickly. It also opens up
trials to those who may have been excluded previously due to distance from
study sites.
Certain populations that are often underrepresented like racial and ethnic
minorities may find virtual models more accessible and convenient. Lower travel
costs and time commitments improve feasibility of participation for many. These
effects could help make clinical trial cohorts more representative of broader
patient communities.
Enhanced Data Capture and Monitoring
Digital technologies enable more sophisticated and continuous data collection
over the course of virtual trials. Remote patient monitoring devices can track
variables like vital signs, symptoms, and medication usage directly in
participants' real-world settings. Integrated mobile apps facilitate frequent
self-reported outcomes and allow two-way communication.
This volume and granularity of digitally-generated data provides researchers
deeper insights into participants' real-time experiences compared to periodic
clinic visits. Adherence and safety can also be monitored more closely through
digital means versus relying on intermittent appointments. Any issues may be
identified and addressed sooner. The data obtained could yield learning
applicable beyond specific trials as well.
Moving Treatment Closer to Patients
One benefit directly experienced by participants is reduced trial-related
burdens. Less time and money spent on travel frees up more hours for work,
school, or other responsibilities. Virtual formats additionally minimize
disease-related barriers that may discourage or prevent involvement otherwise.
For conditions requiring ongoing treatment, the ability to participate from
home brings clinical research participation one step closer.
This enhances opportunities for patients to directly influence and accelerate
development of new therapies. It also gives pharmaceutical sponsors access to a
broader sample of "real world" experiences that better reflect how
interventions may ultimately be used outside of controlled trials. Such insights
could prove valuable for refining products prior to market approval and
commercialization.
Data Privacy and Technology Dependence
Naturally, virtualization introduces new concerns regarding data privacy,
cybersecurity, and technology requirements that must be addressed properly.
Sensitive health information collected and stored digitally requires robust
safeguards. Participants also need reliable access to and proficiency with the
digital tools being utilized in a given study.
Sponsors require IT solutions sophisticated enough to support complex remote
trial workflows while maintaining compliance. Upfront investments may be needed
to develop these capabilities, though payoffs could include lower ongoing
operational costs versus traditional site-based models. With careful planning
and governance, the risks arising from digitization can be responsibly managed.
Growth Projected to Continue
Post-Pandemic
Even as COVID-19 restrictions ease, the transition to virtual clinical trials
seems poised to continue accelerating. Regulators have formalized acceptance of
remote and hybrid methodologies, creating a supportive policy environment.
Sponsors and CROs have demonstrated virtual can achieve objectives like
traditional formats. Participants have also shown openness to engaging
remotely.
As virtual capabilities mature through applications during the ongoing public
health crisis, sponsors are eager to capitalize on the approach's time and cost
efficiencies at scale. Remote participation addresses accessibility limitations
exacerbating underrepresentation challenges as well. Hybrid blends of in-person
and virtual activities also introduce potential for optimizing processes. The
pandemic has served as a forcing function driving real-world adoption of a
transformation whose impact on drug development was long foreseen.
In summary, virtual clinical trials offer a promising means of sustaining and
enhancing the pace of pharmaceutical innovation through inclusive, data-driven
models of research participation undertaken closer to patients. With diligent
stewardship of participants' privacy and effective technology integration,
virtualization aims to revolutionize how treatments are explored, developed and
delivered for 21st century healthcare.
Get
more insights on This Topic- Virtual
Clinical Trials
Explore
More Articles - Fiber
Supplements Market

Comments
Post a Comment